RMC Clinical Trials Available at MD Anderson Cancer Center
- August 20, 2018
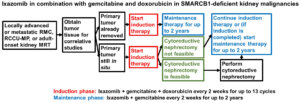
Dr. Msaouel has prepared a trial schema for physicians to know the scheduling of the trial testing the combination of the proteasome inhibitor ixazomib (targeted therapy) with gemcitabine and doxorubicin. The links for the trial are:https://clinicaltrials.gov/ct2/show/NCT03587662 and https://www.mdanderson.org/patients-family/diagnosis-treatment/clinical-trials/clinical-trials-index/clinical-trials-detail.ID2018-0065.html
MD Anderson now has two trials specifically designed for patients with RMC and renal cell carcinoma unclassified with medullary phenotype (RMC in patients without sickle cell trait or disease). The new one above as well as this one testing immunotherapies: https://www.mdanderson.org/patients-family/diagnosis-treatment/clinical-trials/clinical-trials-index/clinical-trials-detail.ID2017-0201.html
Patients with RMC can be enrolled in these trials regardless of whether they have or not sickle cell trait or sickle cell disease (patients without sickle cell trait or disease can also be enrolled).
Contact info for inquiries: 713-792-2830 (Department of Genitourinary Medical Oncology at MD Anderson) as well as Dr. Msaouel’s email: pmsaouel@mdanderson.org
It is critical that we continue to reach our communities and medical personnel through advocacy and education in order to make a difference in the lives of our adolescents and young adults that have been diagnosed with Renal Medullary Carcinoma. Please help us spread the word!
